ABOUT US
Shenzhen Jitong Medical Registration Service Co., Ltd. specializes in providing overseas compliant CRO services for medical device and in vitro diagnostic enterprises. Relying on multinational self operated companies established in multiple countries around the world and experienced professional regulatory teams, we create one-stop solutions for our clients throughout the entire lifecycle, helping to accelerate the smooth launch of products and comprehensively enhance their core competitiveness in the international market. Corporate Vision and Service Philosophy: Adhering to the core service philosophy of "professionalism, efficiency, compliance, and protection", we provide professional regulatory knowledge, efficient service processes, and strict compliance standards to safeguard the internationalization of medical device enterprises. Our corporate vision is to integrate high-quality global regulatory resources, testing resources, and clinical resources, and build an integrated compliance service platform covering the entire lifecycle of medical devices from research and development, testing, registration, clinical trials, to post market launch.
OUR ADVANTAGE
03
Multi-party authentication
Includes: clinical trial research, analytical performance studies, regulatory registration consultation, certification consultation, whitelist registration in various countries, product quality testing, system guidance, regulatory training, local authorized representatives, etc.
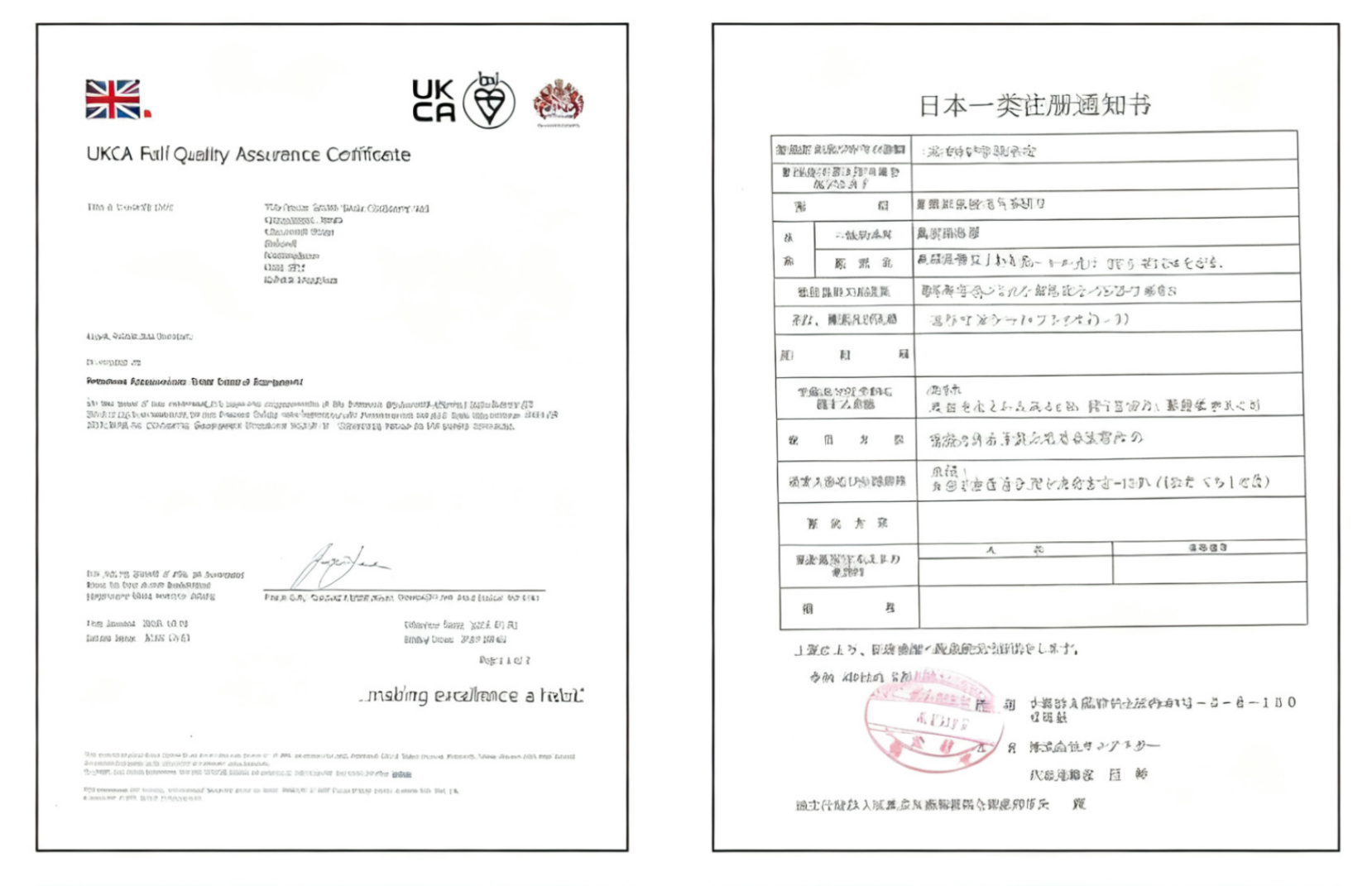
HONOR
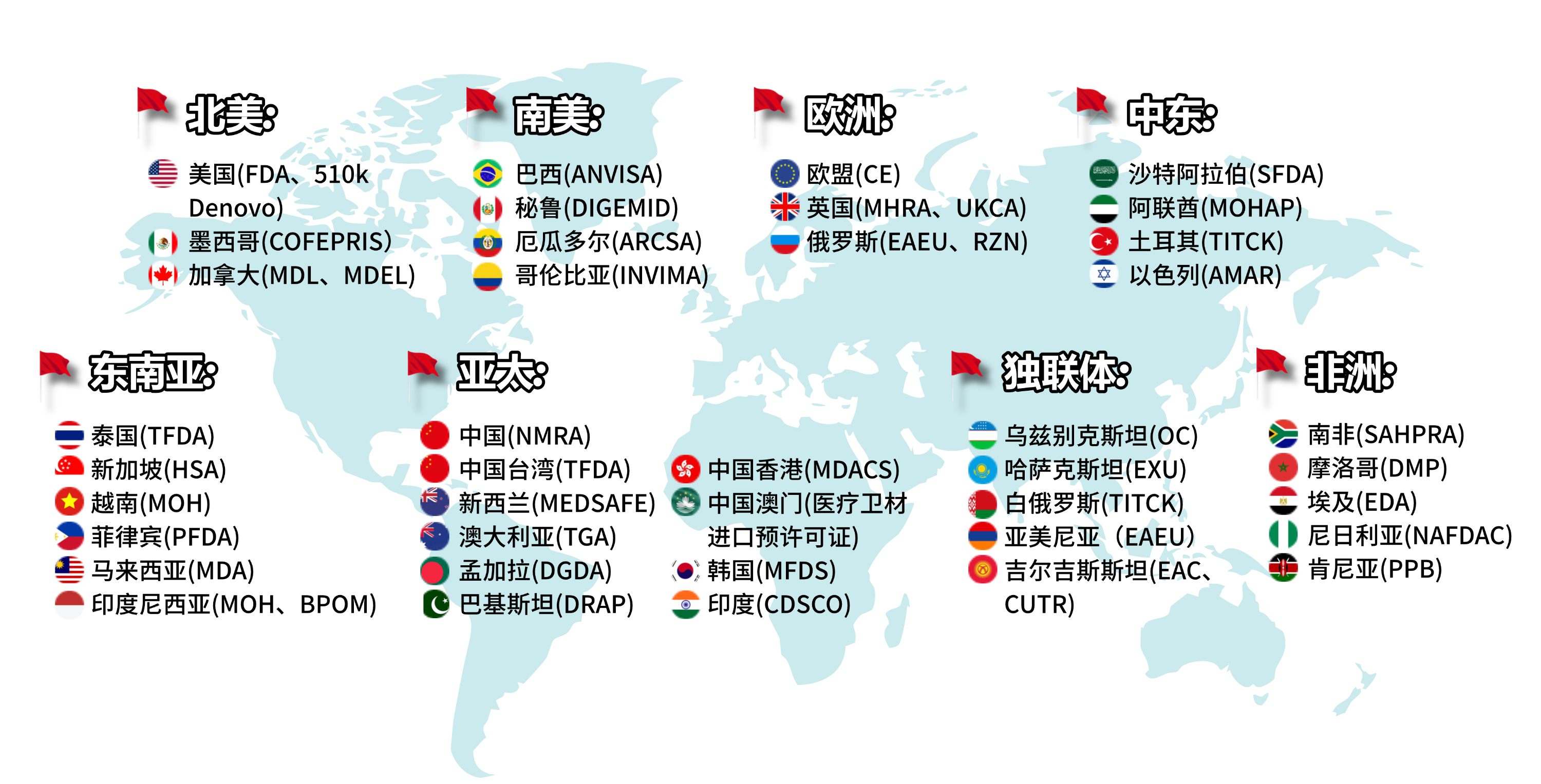
REGISTRATION DISTRIBUTION MAP













.png)
.png)
.png)
.png)